Cross linking is an in-office procedure that involves exposing the collagen fibers of the cornea to ultraviolet (UV) light. There are two different types of procedures: Epithelium-off (epi-off). During this procedure, the epithelium is completely removed to allow the collagen fibers to be fully exposed to the UV light. Epithelium-on (epi-on).. CORNEAL COLLAGEN CROSS-LINKING WITH RIBOFLAVIN AND ULTRAVIOLET-A. Corneal CXL with riboflavin and UVA is a new technique of corneal tissue strengthening using riboflavin as a photosensitizer and UVA to increase the formation of intra-and interfibrillar covalent bonds by photosensitized oxidation. 1 The major indication for the use of CXL is to inhibit the progression of corneal ectasias, such.
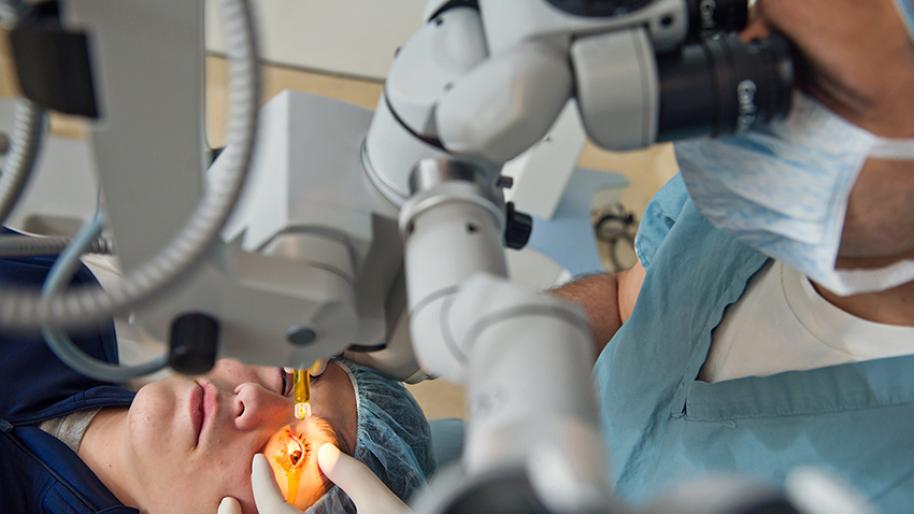
Corneal CrossLinking (Collagen CrossLinking) Kellogg Eye Center Michigan Medicine

Corneal Crosslinking Dr Venter Ophthalmologist Eye South Africa
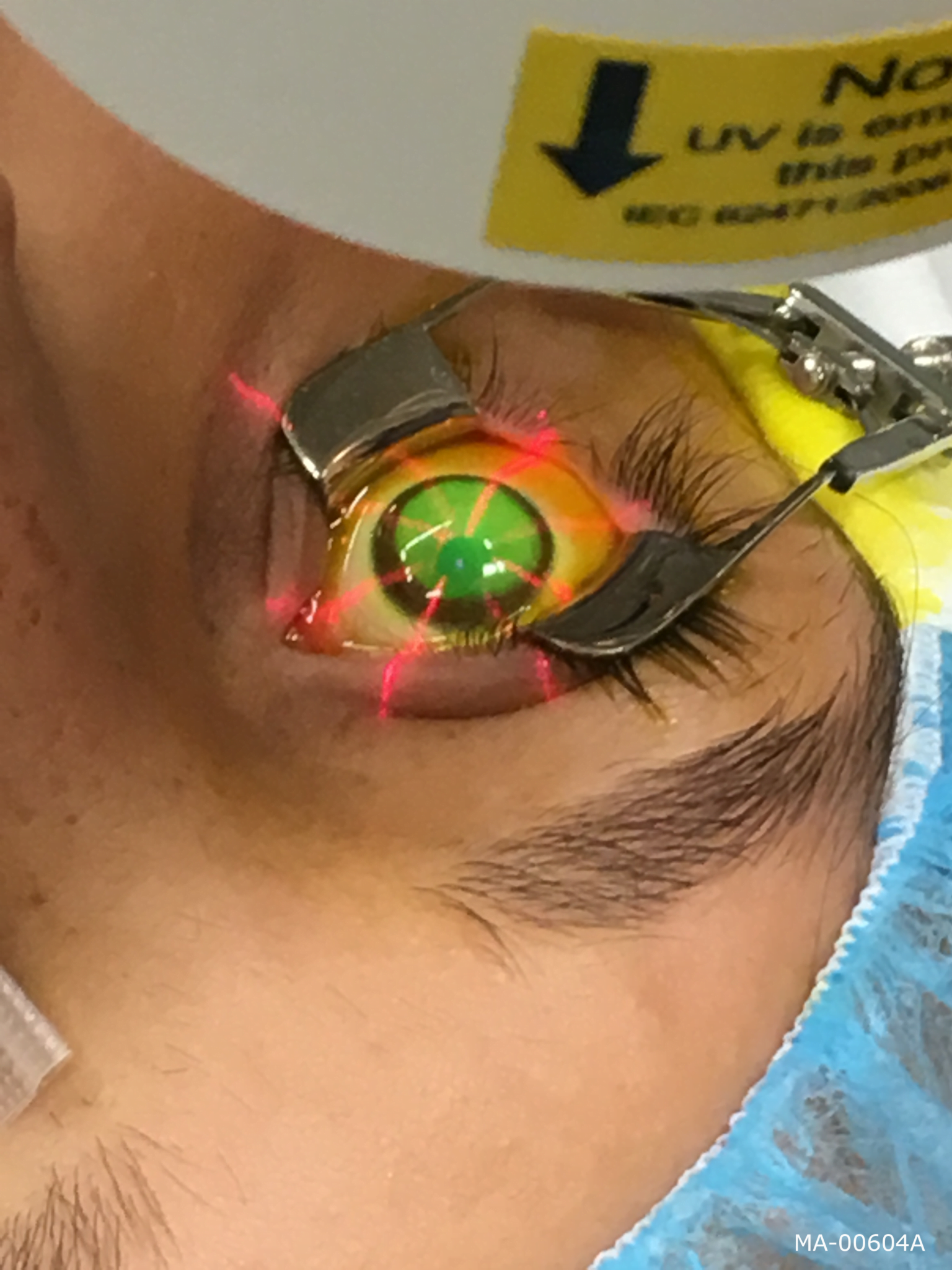
Keratoconus / Corneal ectasia Eye Care Center of Napa Valley

Corneal Cross Linking
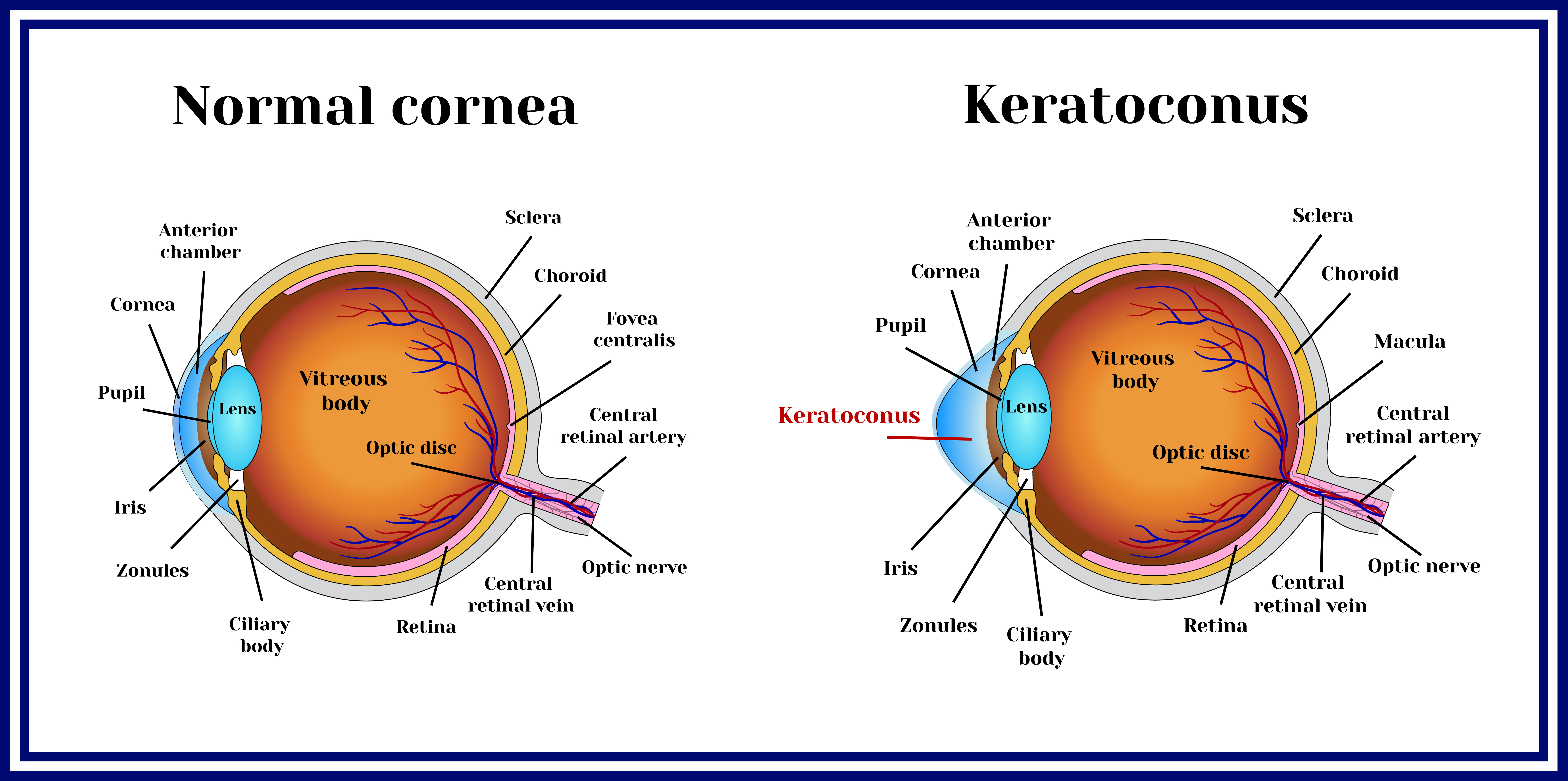
What Is Corneal CrossLinking? (Pros and Cons in 2020) NVISION Eye Centers

Removed corneal epithelium during CXL operation Corneal collagen crosslinking with riboflavin

Crosslinking tratamento para ceratocone Saúde Ocular

Unique solutions for corneal cross linking Servimed Industrial 2020
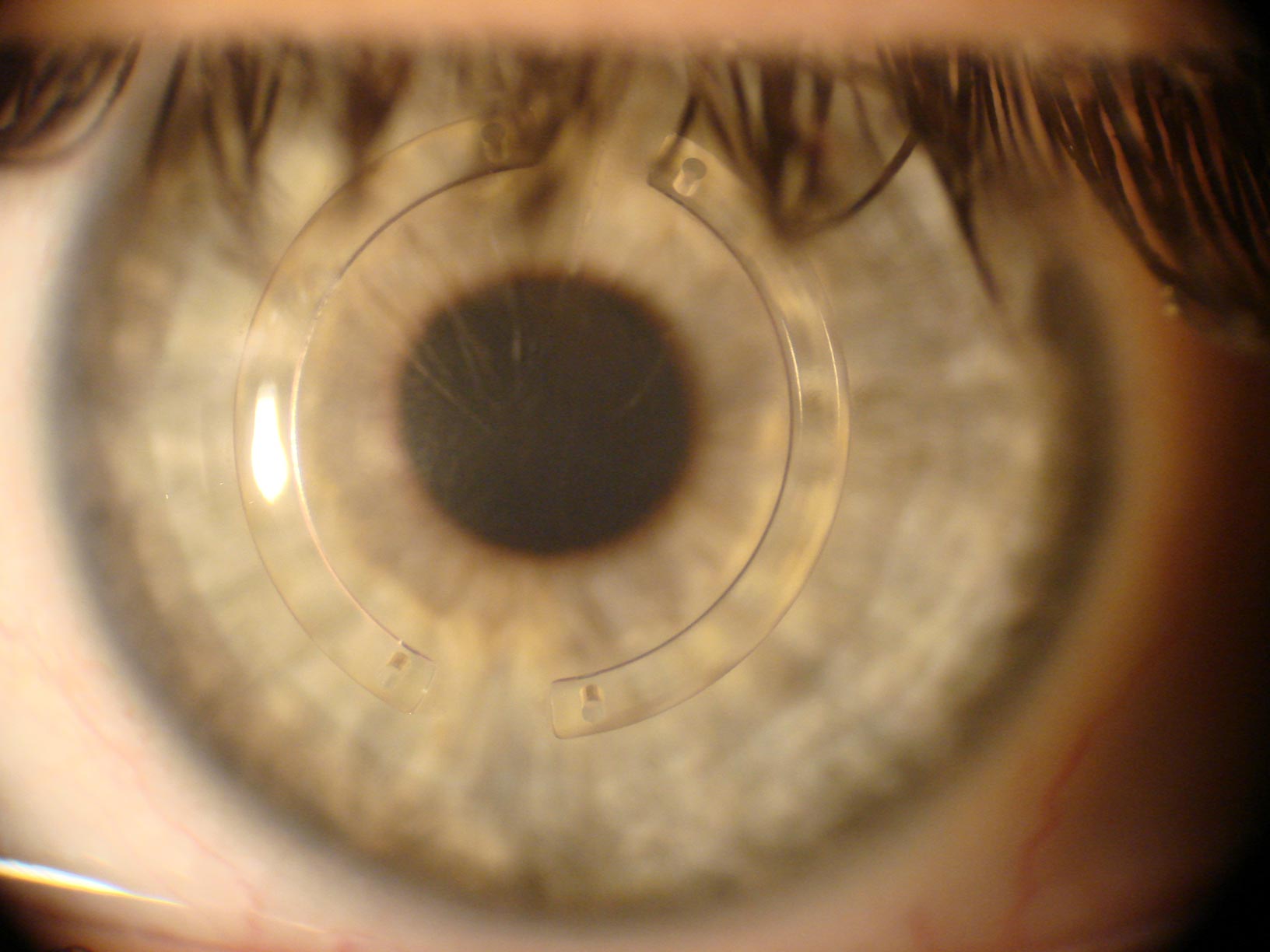
Corneal CrossLinking Dr Shady Awwad, eye surgeon at AUBMC

Corneal Collagen CrossLinking and Femtosecond Laser in Refractive and Cataract Surgery Nova
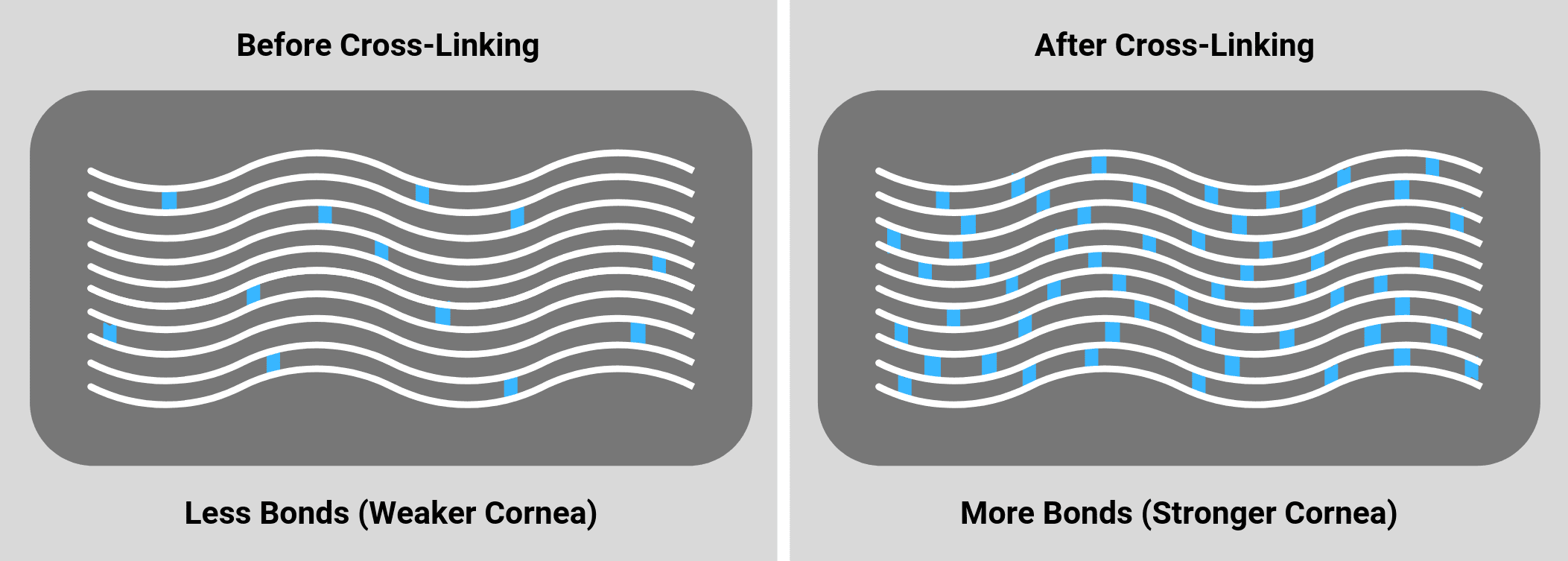
Corneal Collagen CrossLinking
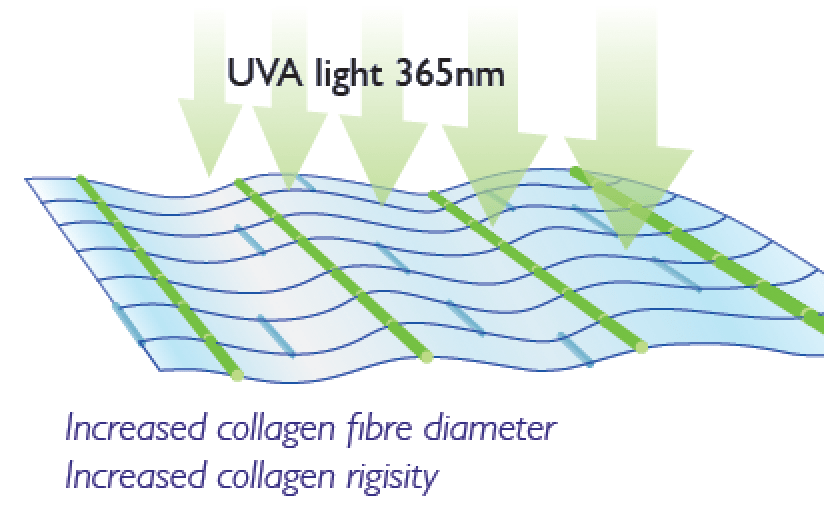
Corneal Collagen Crosslinking Riboflavin CFS

(PDF) Efficacy and safety of transepithelial corneal collagen crosslinking surgery versus

Collagen Crosslinking using Riboflavin and UVA exposure for Keratoconus or C3R treatment
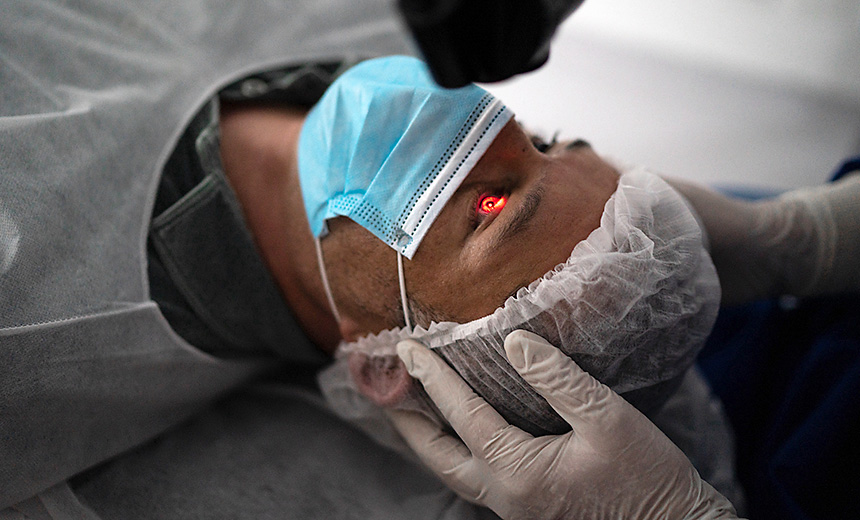
Corneal collagen crosslinking What it is, surgery & more

Corneal Crosslinking Procedure for Keratoconus YouTube

Corneal scarring after corneal collagen crosslinking. Download Scientific Diagram
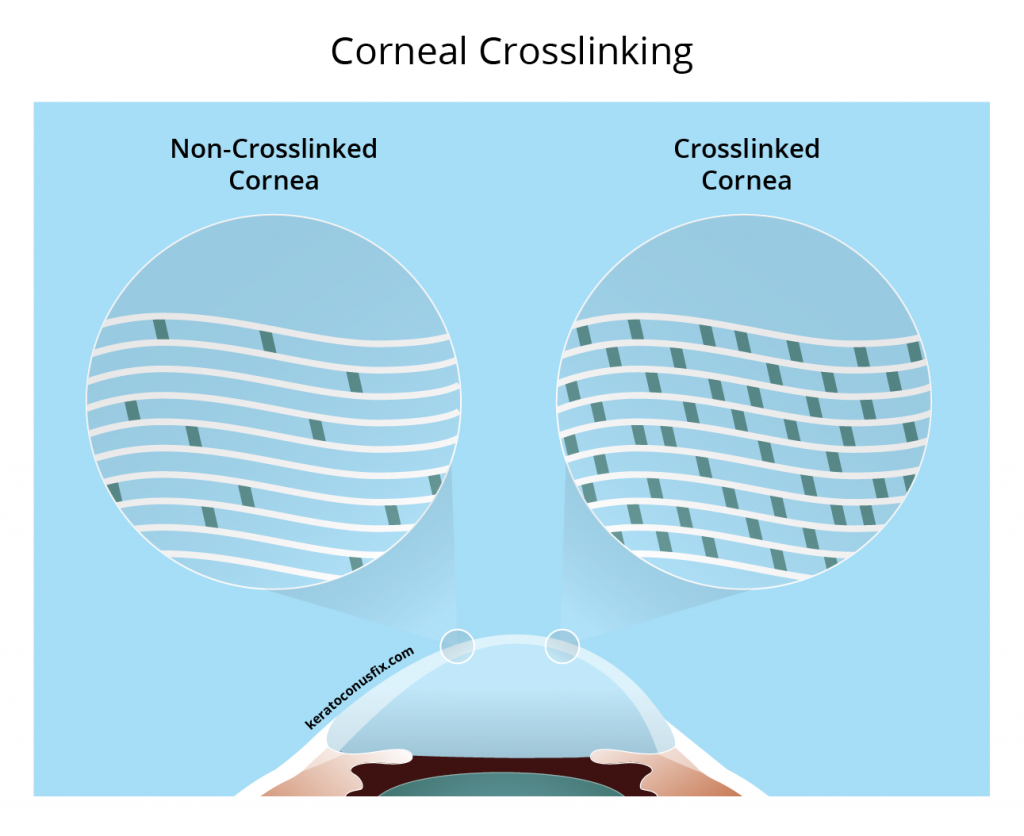
Surgical Treatments for Keratoconus Custom Sclerals

Crosslinking the cornea (CXL) The ELZA Institute, Dietikon, Zurich

cross linking Oftalmontt
Deep anterior lamellar keratoplasty (DALK) has gained widespread utilization in corneal transplantation, thus generating significant interest in the use of tissue adhesives as a substitute for conventional sutures in this surgical procedure. However, several key challenges persist, including prolonged curing times, inadequate mechanical and adhesive properties, and insufficient.. Corneal Collagen Cross-Linking. New York Eye and Ear Infirmary of Mount Sinai (NYEE) is one of a few centers in the New York metropolitan area equipped to perform collagen cross-linking (CXL), a breakthrough technique for treating people with keratoconus and corneal ectasia following refractive surgery. Approved by the FDA in April 2016, CXL.