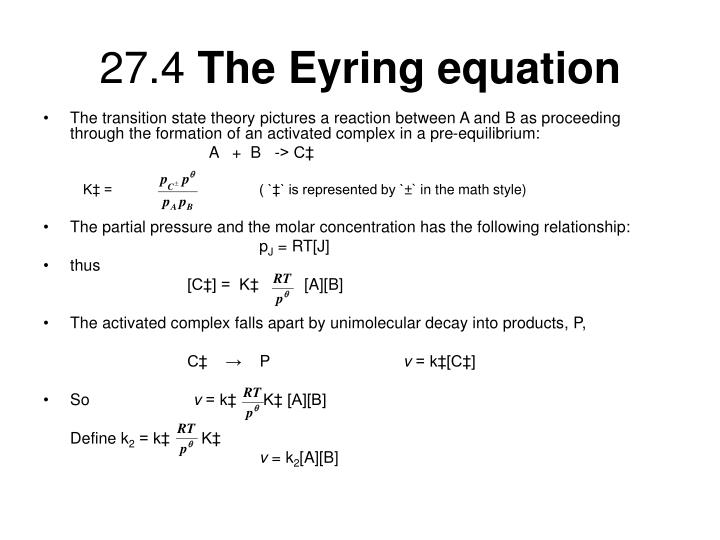
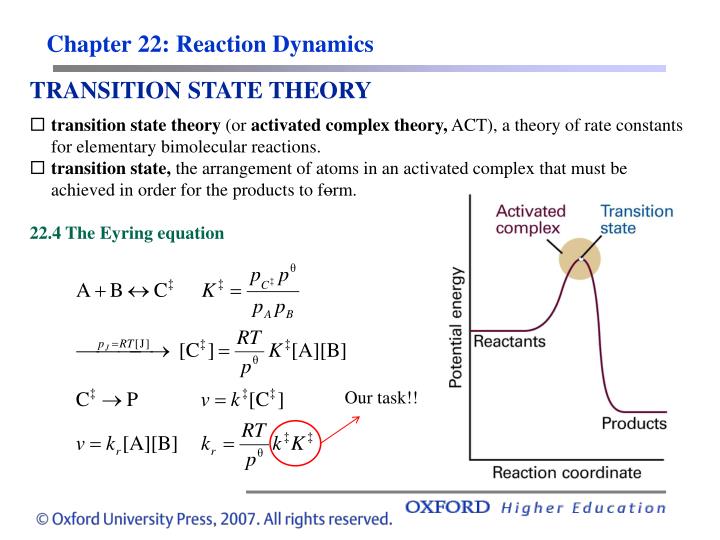
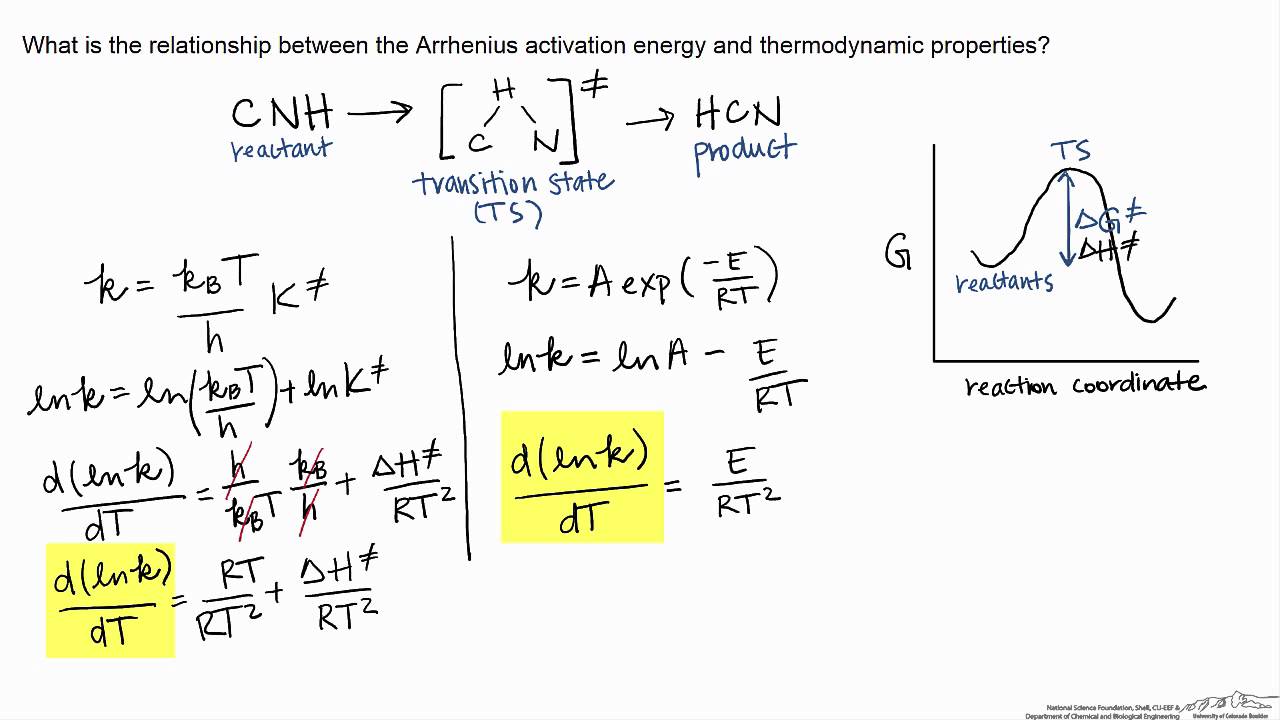
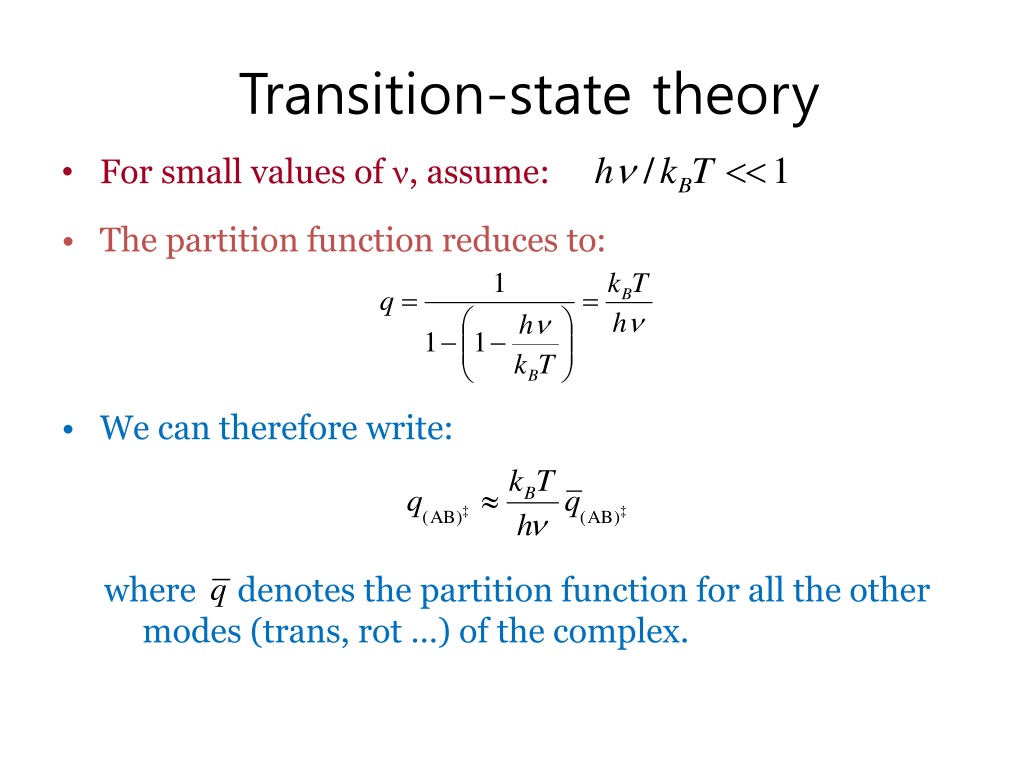
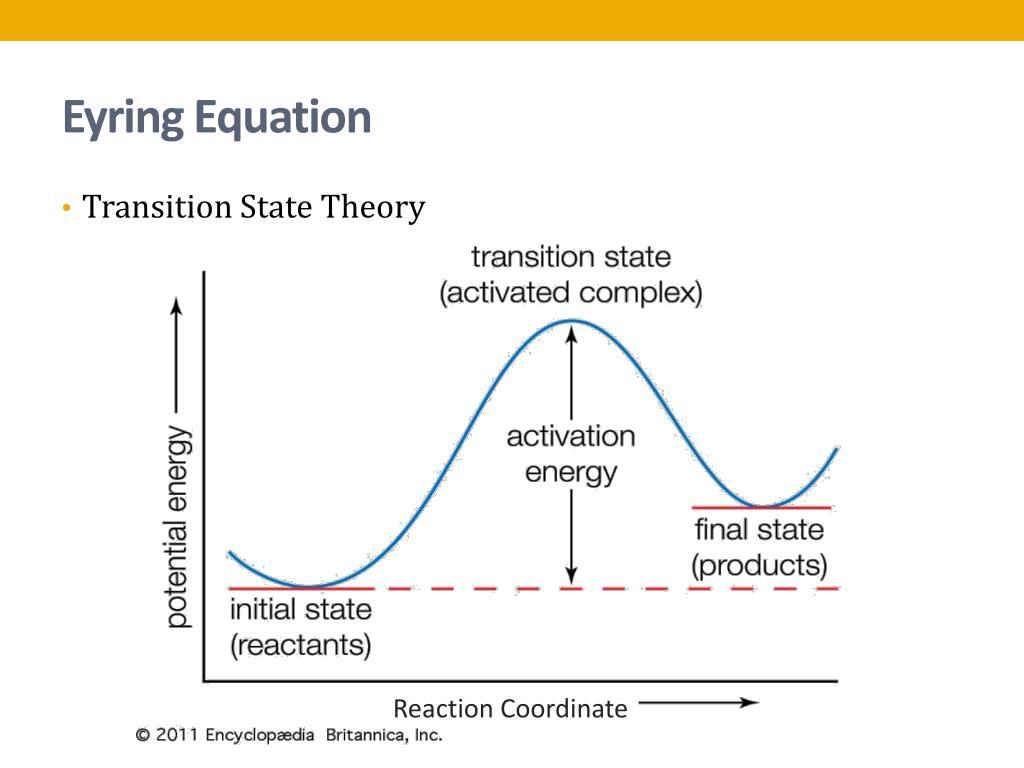
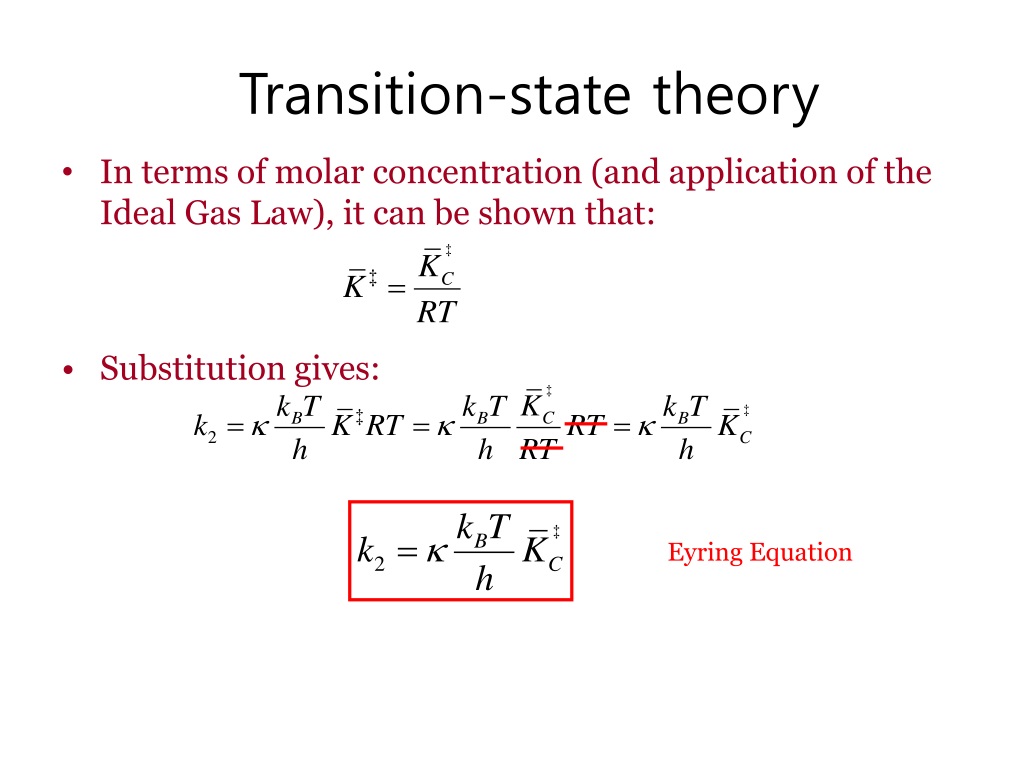
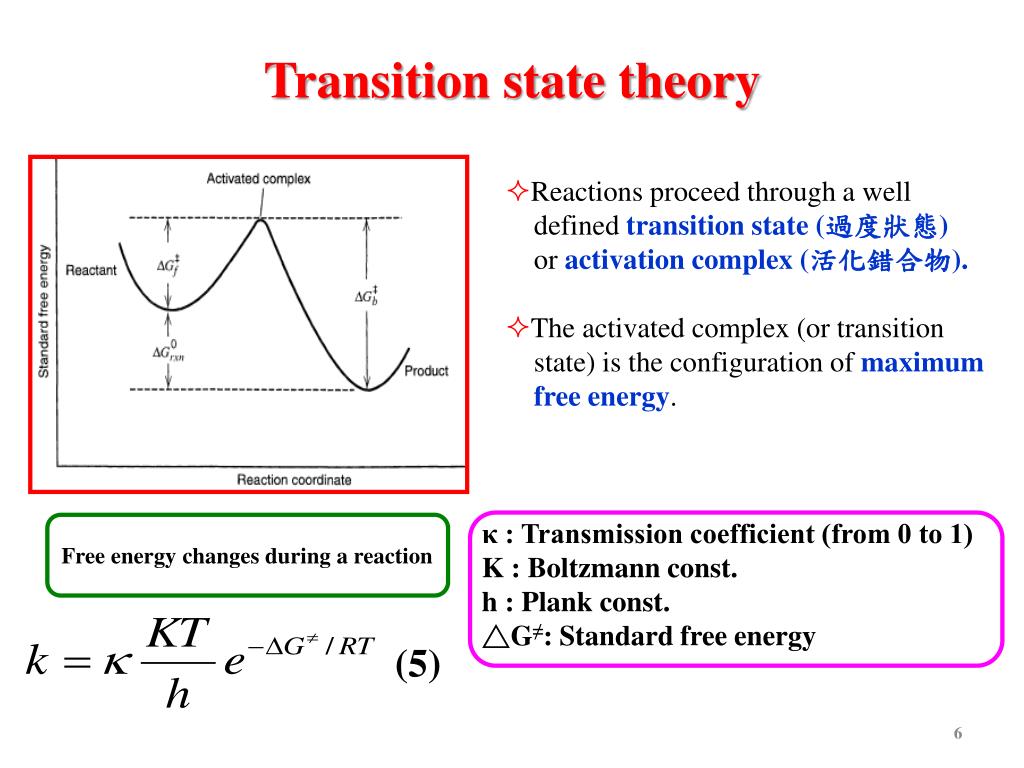
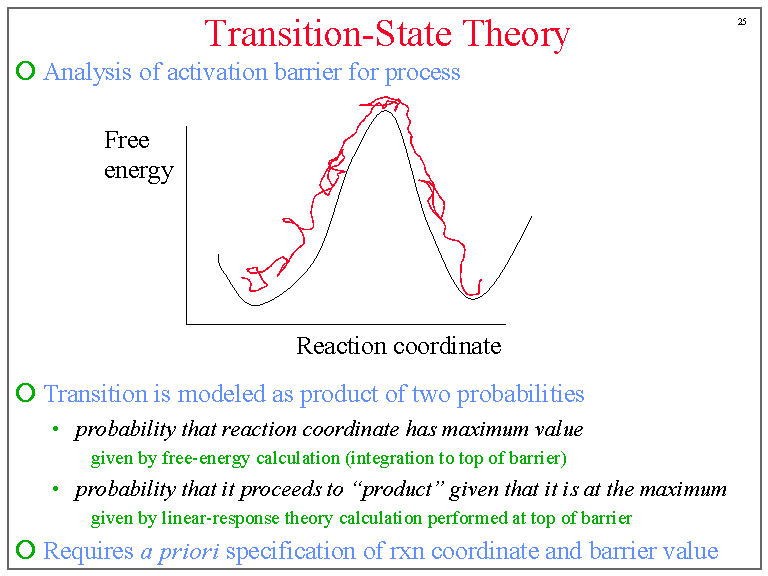
The theory assumes that reactants are hard spheres rather than molecules with specific structures. In 1935, Henry Eyring helped develop a new theory called the transition state theory to provide a more accurate alternative to the previously used Arrhenius equation and the collision theory. The Eyring equation involves the statistical frequency.. Transition state theory relies on three key assumptions in its derivation. Reactants are in constant equilibrium with the transition state structure. The energy of the particles follow a Boltzmann distribution.. The Eyring equation can also be expressed in terms thermodynamic parameters.
PPT Activated complex theory PowerPoint Presentation ID3995760

Transitionstate theory Definition & Facts Britannica
Get Transition State Theory Equation Ariana

PPT Chemical the rates of reactions PowerPoint Presentation ID1523033

PPT Enzymology PowerPoint Presentation, free download ID1794432
Relationship Between Arrhenius Activation Energy and Transition State Theory (Eyring Equation
PPT Chemical the rates of reactions PowerPoint Presentation ID1523033
PPT EXPERIMENT 9 PowerPoint Presentation, free download ID1769598

PPT Chemical the rates of reactions PowerPoint Presentation ID1523033

Transition State Theory Entropy Please read note in comments! YouTube
PPT Chemical the rates of reactions PowerPoint Presentation ID1523033

Eyring analysis of transitionstate thermodynamics Linear Eyring plots... Download Scientific
Get Transition State Theory Equation Ariana
TransitionState Theory

Transition State Theory Enthalpy YouTube
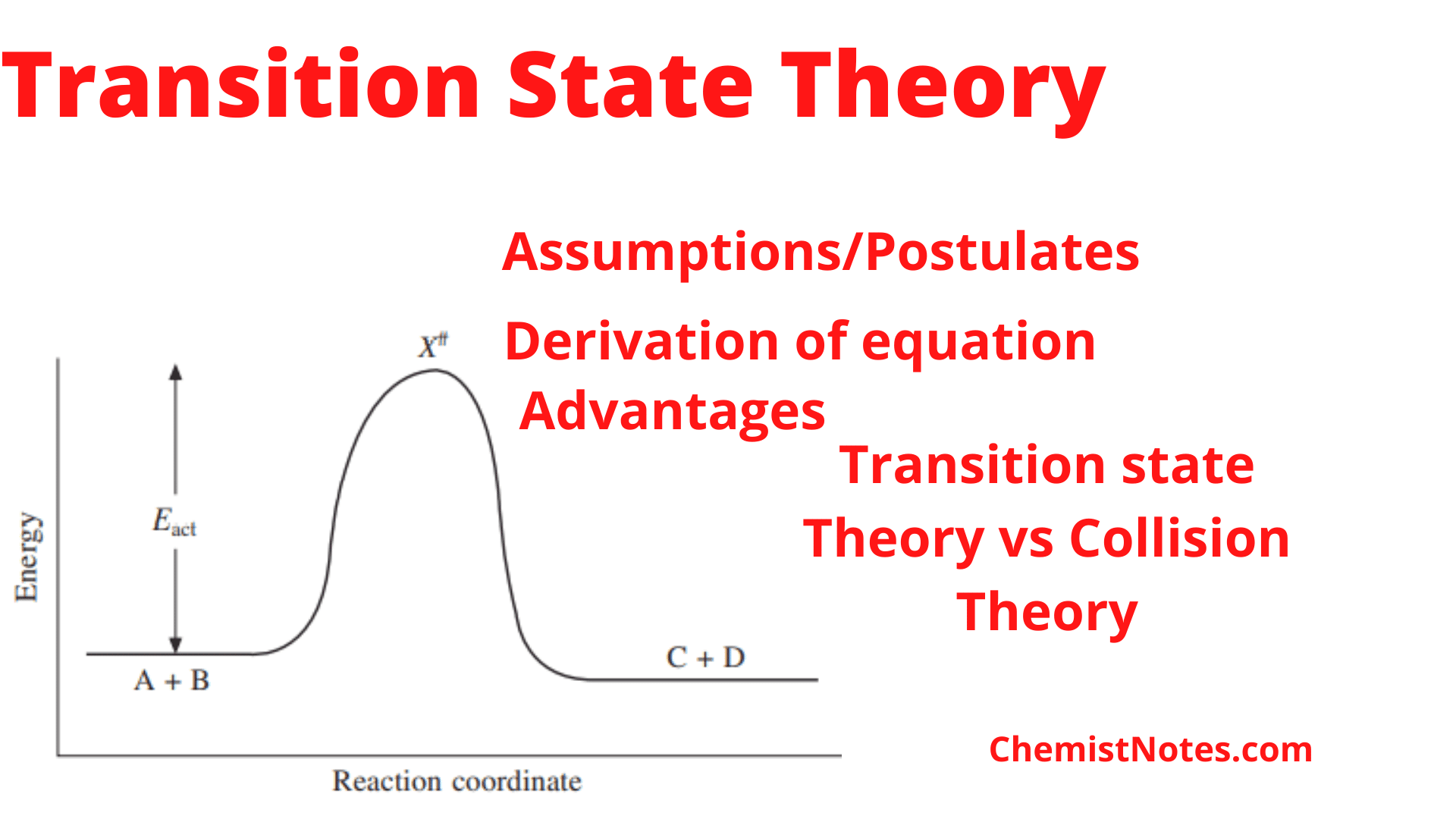
Transition state theory Postulates, equation and Collision theory vs transition state theory

Transition State Theory Gibbs Energy YouTube

TRANSITION STATE THEORY ACTIVATED COMPLEX THEORY EYRING EQUATION CHEMICAL YouTube

Transition State Theory Activated Complex Theory Eyring Equation Chemical for CSIR

PPT Chemical the rates of reactions PowerPoint Presentation ID1523033
California State University East Bay. Transition state theory was proposed in 1935 by Henry Erying, and further developed by Merrideth G. Evans and Michael Polanyi (Laidler & King, 1983), as another means of accounting for chemical reaction rates. It is based on the idea that a molecular collision that leads to reaction must pass through an.. Transition-state theory allows for the characterization of kinetic processes in terms of enthalpy and entropy of activation by using the Eyring equation. However, for reactions in solution, it fails to take the change of viscosity of solvents with temperature into account.